Learn more about the NPX product development process
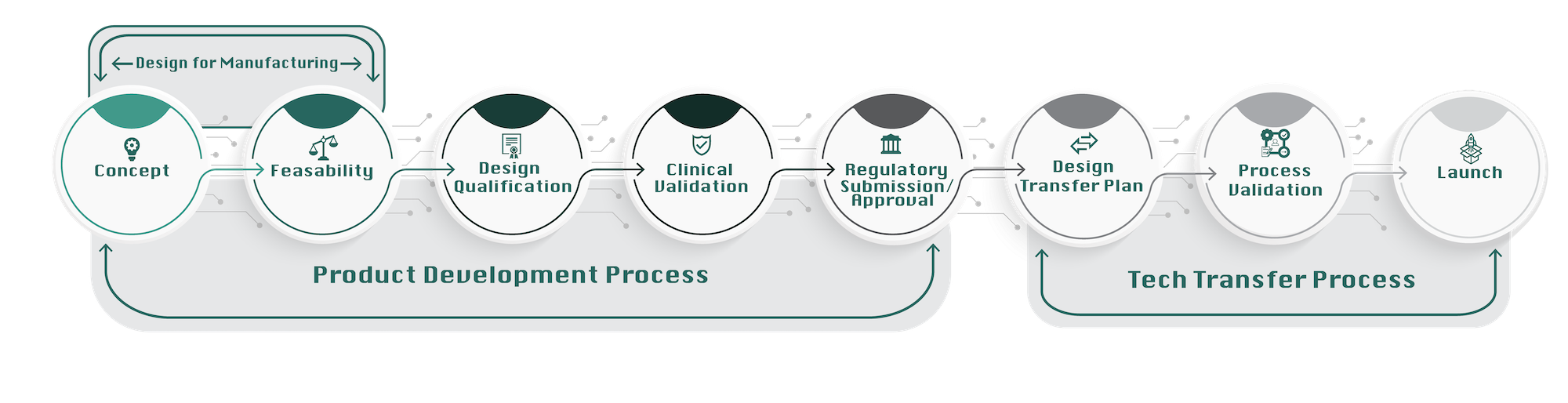
Concept
The process always begins with an idea–a concept comes to mind typically by way of needing to solve a real-world problem. Defining the problem can often save a project immense time and money, as there have been many good products developed that unfortunately did not solve the right problem.
Feasibility
Not all ideas are made equal and validating ideas requires taking the time to determine if they are feasible. Making a couple designs and proving the theory before larger resources are allocated is what we do.
Design Qualification
Once an idea has become a viable product, greater testing must commence through validating the design of the product. Making one is easy, making thousands a month is something different, and the discipline needed to make this transition is what NPX brings to the partnership.
Clinical Validation
Medical devices are different from other categories of products in that the device design must pass muster not only from a marketability standpoint, but also clinically. Years of experience in clinical cases have honed NPX’s skill in successful clinical rollout and trial completion.
Regulatory Submission and Approval
If a product remains viable through all of the previous stages, it must also stand up to the scrutiny of regulatory requirements. We can help navigate all the requirements for your submissions, providing all necessary data to satisfy the regulatory bodies.
Technology Transfer Process
Design Transfer Plan
Now that the device/product has been submitted and approved, the technology transfer process can begin. This starts with transferring the design itself.
Process Validation
In order to manufacture the product, the process must pass a rigorous validation phase.
Launch!
Once all validations have passed, the time of truth has arrived. The launch phase can begin!


