Medical Device
Prototype to Production
With decades of combined experience NPX understands the challenges of product development. We are equipped to advance your project quickly with our rapid prototyping and engineering services with no minimum order. As you approach commercial release our team can grow with you and help you with your production volume needs. Our goal is to be a true partner throughout the process.
Medical Device
Research and Development
We provide our insights and subject matter expertise to help you through the complete design and development process.
Our team will work to create a very specific plan with the commercialization of your product in mind from start to finish.
Medical Device
Rapid Prototyping
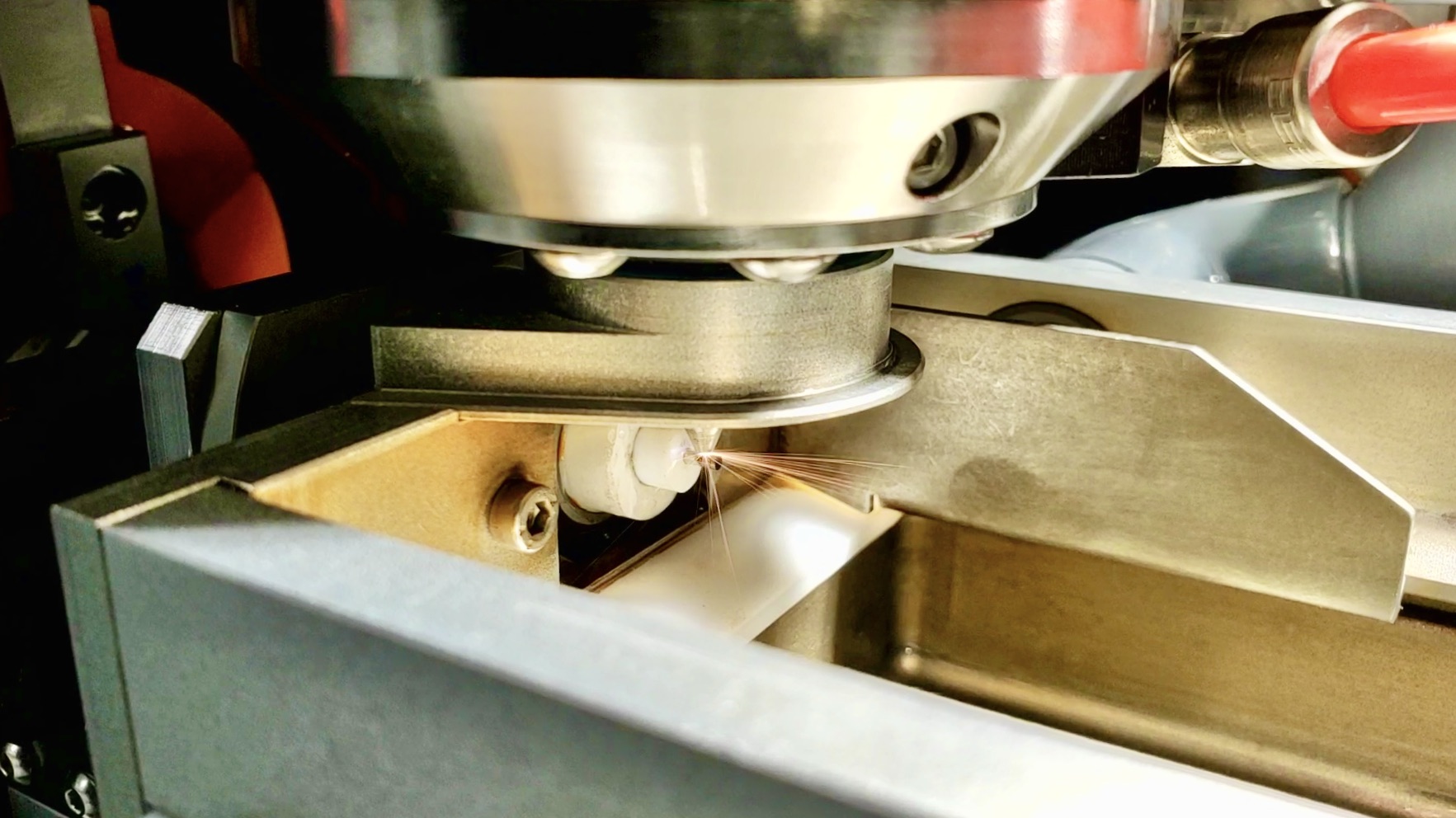
We pride ourselves in fueling fast, efficient, and accurate product iteration. It’s not uncommon for us to produce parts before other companies have produced a quote.
We help communicate concepts, prove out theories, refine ideas, and provide the product to help the project move forward.


The Nextern Difference
As a division of Nextern, we provide world-class design, prototyping, optimization and manufacturing capabilities to enable medical devices and drive technology forward.
Could we send you a free, no-obligation quote for your project?
Have a question? We are happy to help.
Please feel free to take advantage of our FAQ’s, or contact us directly.
Frequently Asked Questions
How quickly can NPX rapid prototype parts?
We pride ourselves in fueling fast, efficient, and accurate product iteration. It’s not uncommon for us to produce parts before other companies have produced a quote.
While each project is unique, the typical turnaround from NPX is 3-5 business days for prototype orders.
How is NPX able to turn around parts so quickly?
NPX continues to reserve excess capacity so that we can respond quickly. This means we have the capacity ready to turn on at a moment’s notice, allowing lead times to stay short.
As medical device engineers ourselves, this is incredibly important to us to maintain as we continue to grow.
We know how important it is to have your R&D or production product produced without worry.
What market areas does NPX have experience engineering and/or manufacturing products for?
We have extensive experience in most fields of medical devices – including but not limited to: Cardiovascular, Cardiac Surgery, Interventional Radiology, Vascular Surgery, Otolaryngology, Urology, Neurovascular, Orthopedics, Gynecology, Thoracic Surgery, and Gastroenterology.
How is engineering consulting billed? Do we need to sign a large contract?
We offer engineering consulting and we do it on a time and materials basis reducing our customer’s tendencies to otherwise over plan and overpay.
Driven by experience, we are very confident in our ability to meet our customer’s needs, so we often offer to start with a 20 to 40-hour block of time to meet with our team and when things go well, we move forward incrementally–removing the need to sign large contracts.
This is a win-win scenario as it allows our customers to get comfortable with us without making a massive time or financial commitment and it allows us to have a very clear understanding of our customer’s goals and expectations.
Does NPX work with small medical device companies and start ups?
Many larger contract manufacturing companies tend to sort their customers based on the bottom line–the dollars they are bringing to the table. We’re trying to meet the needs of all of our customers–even the smallest ones.
We have found that there is a pent up demand in this category–that we are quickly able to meet.
This approach has allowed us, in some areas of our business to double or even triple our capacity.
We can often bring the resources the customer needs to the project reducing overall project cost, engineering, testing, quality, manufacturing, and distribution.
Does NPX have minimum project requirements?
In the medical device industry, it is commonplace for small companies to be priced out of the market almost immediately during an initial iteration.
When some large contract manufacturing companies won’t consider your project unless you are willing to come to the table with a considerable project minimum, it is very difficult to get any traction. Not to mention, these same companies want minimum orders for every subsequent iteration.
NPX is different. We do not put any minimum project requirements in place. We’re here to serve you.
Does NPX have ISO certification?
Yes. NPX is ISO 13485:2016 certified.


