
New medical devices are always in the works. The biomedical industry is evolving in ways that are increasingly focused on optimizing healthcare and holistically improving the patient experience. For example,…

New medical devices are always in the works. The biomedical industry is evolving in ways that are increasingly focused on optimizing healthcare and holistically improving the patient experience. For example,…

The process for bringing a medical device to market starts with an initial concept and can last years before landing in the hands of an end-user. During each phase of…

What do you do if you have an idea for a new medical device or instrument, but don’t know how to get it in the hands of caregivers and patients?…
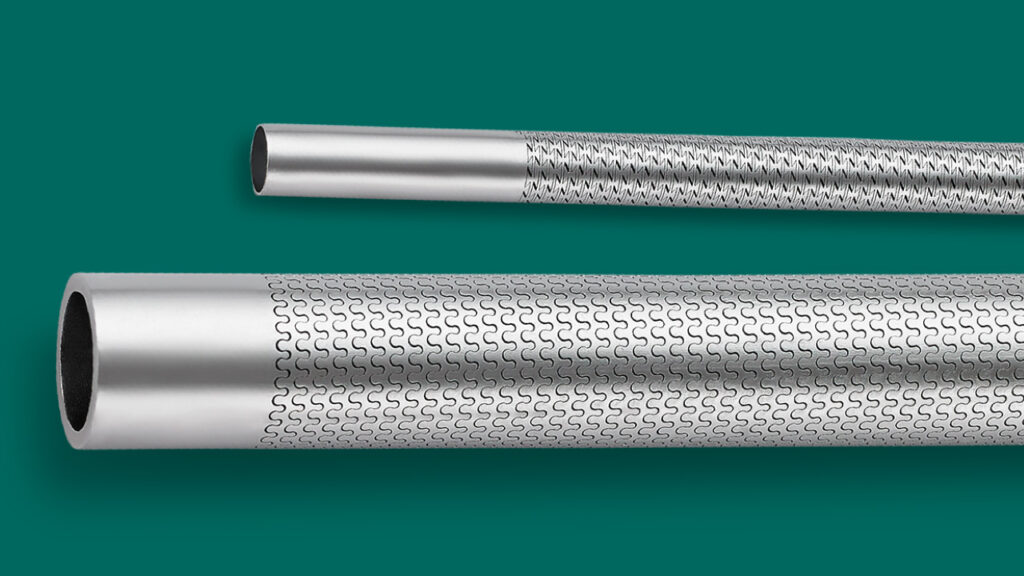
Medical device design is a process that demands months or sometimes years. The industry’s tendency to navigate technology evolution and ongoing developments dictates any given medical device project’s scope, goals…

The development process for medical devices is rigorous and extensive to ensure a new device or instrument is ready for patient or provider use. Part of that process includes prototyping…

NPX Medical has partnered with Nextern, Inc., a global medical device development and manufacturing enterprise headquartered in White Bear Lake, Minnesota. NPX brings our full life cycle offerings to Nextern….

The process of creating a new medical device often begins with careful planning, including research and development. Medical device research and development is a key first step in the manufacturing…
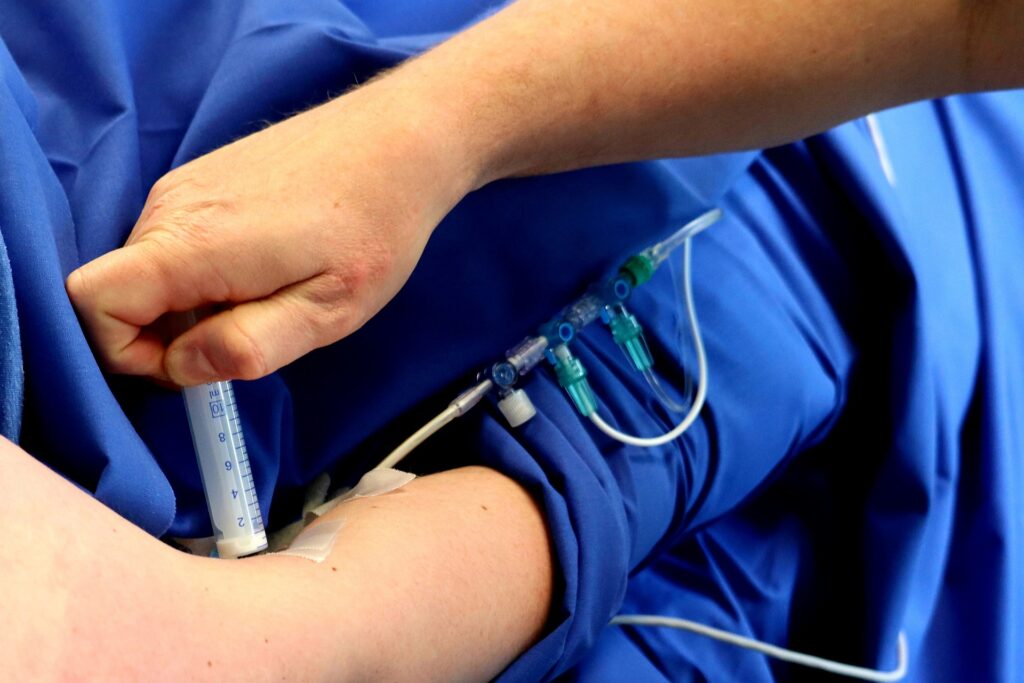
During the prototyping phase, your medical device uses different materials and processes for effective testing and optimizing. Medical equipment such as catheters, stents, tubes and more require durable yet flexible…

Developing medical devices and equipment requires extensive knowledge and experience of the field. At NPX, we have put in the time and research to understand what developers, patients, doctors and…